The Beef or Pork Tapeworm Can Be in Length
Continuing Education Activity
Cestodes are flat, parasitic, hermaphroditic tapeworms with complex life cycles that infect animals, including humans. Three cestodes that cause human disease are Taenia solium (pork tapeworm), Taenia saginata (beef tapeworm), and Diphyllobothrium (fish tapeworm). This activity reviews the evaluation and treatment of these tapeworm infections and highlights the role of the interprofessional team in evaluating and treating affected patients.
Objectives:
-
Describe the transmission of Taenia solium, Taenia saginata, and Diphyllobothrium.
-
Outline the history and physical exam findings expected in patients with Taenia solium, Taenia saginata, and Diphyllobothrium infections.
-
Summarize the treatment options for Taenia solium, Taenia saginata, and Diphyllobothrium infections.
-
Review the importance of collaboration and communication amongst the interprofessional team to enhance the delivery of care for patients with Taenia solium, Taenia saginata, or Diphyllobothrium infections.
Access free multiple choice questions on this topic.
Introduction
Cestodes are flat, parasitic, hermaphroditic tapeworms with complex life cycles that infect animals, including humans. Although there are multiple species of cestodes, this paper will focus on three particular species that cause human disease: Taenia solium (pork tapeworm), Taenia saginata (beef tapeworm), and Diphyllobothrium (fish tapeworm).
There are two types of infections that can develop depending on the route of infection. The first type is caused by ingesting the eggs from an adult worm (expelled in animal or human feces) which results in developing cysticerci (larval stage) within the animal or human tissues. This is known as cysticercosis. If these cysticerci develop in the central nervous system, this is called neurocysticercosis. The second type is due to ingesting the cysticerci themselves, from poorly cooked infected meat, which results in the growth and infestation of the adult tapeworm within the gastrointestinal tract. The infection with the adult tapeworm is called taeniasis if infected by the Taenia family or diphyllobothriasis if infected by Diphyllobothrium.
These tapeworm infections have significant human and veterinary disease implications as well as economic effects.[1][2] Neurocysticercosis is an important cause of seizures and accounts for approximately 30% of all epilepsy cases in most developing countries.[1][3][4]
Etiology
Tapeworms require single or multiple hosts to complete their life cycle. Infection of the host depends on the stage of the life cycle the tapeworm is in when the host becomes exposed.
For diphyllobothriasis, humans become infected by consuming undercooked or raw fish infected with the worm larval stage (cysticerci).[5] The worm attaches to the intestines, matures to the adult form, and releases eggs in human (or other fish-eating carnivores) stool. The infected stool, due to poor hygiene conditions or lack of appropriate sanitation, can contaminate water where the eggs hatch and are consumed by their first intermediate host, copepods (small crustaceans). Fresh or saltwater fish ingest these copepods as the second intermediate host, which are then consumed by humans. Most commonly these fish include perch, pike, burbot, whitefish, salmon, and trout.[5] Once a person consumes raw or under-cooked fish, the larva attaches to the small intestine and becomes a mature tapeworm in 5 to 6 weeks.[6] The most common species for infection is D. latum; however, with improved molecular testing, other species are being recognized as pathogenic.[7]Diphyllobothrium may live for decades in the human host and continue to pass eggs through their lifetime.[6]
Taeniasis has a similar reproductive cycle as Diphyllobothriasis. Eggs expelled in animal or human stool contaminate the water and vegetation that cattle or swine ingest. Eggs or gravid proglottids (worm segments) are ingested and penetrate the intestinal wall to infiltrate various striated muscles or other tissue and develop into cysticerci. If humans ingest undercooked pork (T. solium) or beef (T. saginata) with cysticerci, the worm will attach to the jejunum and mature to an adult worm in 5 to 12 weeks for T. solium and 10 to 12 weeks for T. saginata.[6] The adult tapeworm may live for 25 years or the lifespan of the host and can infect thousands of cattle or pigs over the lifetime of the tapeworm.[2][6]
Ingestion of cysticerci only causes taeniosis or the intestinal adult tapeworm infection; ingestion of cysticerci does not cause cysticercosis, which is due to the direct ingestion of T. solium eggs passed in the stool due to poor hygiene practices.[3] Autoinfection is possible through this route when an infected person with taeniasis ingests passed Taenia eggs. Embryos hatch in the small intestine then may invade and spread hematogenously to the central nervous system (neurocysticercosis) or muscle and other tissue. Person-to-person spread is more common than previously thought rather than contamination through environmental sources.[3]
Epidemiology
Taeniidae have a worldwide distribution except forTaenia asiaticathat appears to be restricted to Asian countries.[1][6] Although Taenia solium andTaenia saginata have a global distribution, the highest prevalence is found in areas with poor access to adequate clean water and sanitation and areas that ingest raw or under-cooked meat.[8]T. solium has been effectively controlled in most of Europe, North America, and Australia while the highest prevalence is found in Africa, Asia, Latin America, East Europe, China, Pakistan, and India.[1][3][6] It is important to note that due to potential diagnostic limitations, the prevalence of neurocysticercosis is likely under-diagnosed.[9]T. saginata has its highest prevalence in Southern and Eastern Africa.[8]
Diphyllobothriasis has a global distribution with some decline in human disease in North American, Asia, and most of Europe but also a reemergence in some areas, including Russia, South Korea, Japan, and South America.[5] Diphyllobothriasis is generally associated with cold waters in the Palaearctic region, although some cases have been reported in South America. The occurrence is unknown in Africa and Australia.[5]D. latum is thought to be the most common cause of Diphyllobothriosis; however, recent studies show that due to the difficulty in classifying species, D. latum may be misdiagnosed as other emerging species. D. latum is mostly reported in northern Europe, Russia, and North America; however, it also was recently found in South America (Chile).[7]
History and Physical
Infection with the adult tapeworm is most commonly asymptomatic or cause mild disease. Rarely do sequela from the intestinal parasites occur.[6]
Taeniasis typically will cause few or mild health concerns but is characterized by anal pruritus from the escaping proglottids (worm segments) and mild abdominal pain.[8] Most patients become acutely aware of the infection by noting the passage of proglottids in their feces and from the anal pruritus.[2] On rare occasions, patients may regurgitate or aspirate a proglottid.[6] Other symptoms include nausea, change in appetite (both increase and decrease have been reported), weakness, weight loss, and less commonly headache, constipation, dizziness, and diarrhea.[2][6] Children are often more symptomatic than adults.[6] Rare complications of the intestinal worm include intestinal, biliary, or pancreatic obstruction requiring surgical intervention.[2][6] Tapeworms in the intestinal tract cause a poor inflammatory response due to their limited contact with the mucosal tissue, which may explain the limited symptomology in most patients.[2]
Diphyllobothrium is one of the largest parasites of humans and may grow up to 2 to 15 m in length. Despite its size, Diphyllobothrium presents as either mild disease or asymptomatic. One in five infections may have diarrhea, abdominal pain, fatigue, constipation, pernicious anemia, and less commonly headache and allergic reactions.[5] Bloating, sore tongue, sore gums, loss or increased appetite have also been reported.[6] Similar to taeniasis, migration of these worms can rarely cause intestinal obstruction or cholecystitis, and proglottids can be seen passed in the stool or regurgitated.[5][6] Prolonged or large D. latum burden may cause megaloblastic anemia due to the parasite absorbing up to 80% of B12 uptake within the gut.[5] Although approximately 40% of patients with the worm infection have decreased B12, only 2% develop anemia.[6]
Cysticercosis also has a fairly unremarkable presentation given that cysts in most tissues are generally asymptomatic and rarely noticed. Human cysticercosis may affect multiple tissues; however, the parasite is destroyed by the host immune response and surviving only in areas impervious to major immune responses such as the central nervous system and the eye.[9] Cysts are most common in the muscular, subcutaneous, ocular, and CNS sites.[1] CNS involvement (neurocysticercosis) has the highest morbidity while larval cysts elsewhere are usually destroyed leading to asymptomatic disease.
Neurocysticercosis (NCC) symptoms can range from asymptomatic brain lesions to mimicking any kind of neurological disorder.[3] Symptoms include seizures, focal neurological deficits, intracranial hypertension, cognitive decline, vague symptoms of a headache, associated stroke, or involuntary movements. Symptomatology often depends on the location of the brain lesions; for example, intraparenchymal cysts more often leading to seizures and headache, while cysts in the subarachnoid spaces and ventricles lead to hydrocephalus.[4] NCC is commonly associated with seizures and epilepsy; however, the risk of developing epilepsy is unclear. Many people with NCC may develop epilepsy, but the rates are highly variable and can be anywhere from 10% to 50%.[9]
Evaluation
Evaluation of a patient with the adult tapeworm infection relies mostly on microscopic fecal examination searching for the presence of eggs in stool. Although this allows for the diagnosis of the genus of the parasite, it often does not distinguish between species. Serum and fecal molecular testing can aid in the diagnosis and species differentiation. NCC requires a combination of molecular tests and imaging. Eosinophilia is often present but rarely will rise more than 50%. An increase in 1% to 15% is more likely, and serum IgE levels may be increased as well.[6]
T. saginata and T. solium eggs can be identified but not differentiated by microscopic fecal examination.[2] The species may be distinguished by a passed proglottid based on the number of uterine branches.[6] Stool examination may be less effective in the case of T. saginata since gravid proglottids often emerge spontaneously through the anus, depositing eggs on the perianal and perineal regions.[6] Therefore, an anal swab may be recommended for the recovery of ova. Regardless, Taenia diagnosis relies on direct microscopy of expelled eggs in feces despite the low sensitivity given the intermittent nature of eggs shedding (sensitivity ranges from 3.9% to 52.5%).[1] Copro-Ag ELISA detection (from stool samples) does not depend on active shedding of eggs for positive detection. However, this cannot distinguish between species, and there may be cross-reactivity with other parasites including Ascaris, Trichuris, Hymenoplepis nana, and protozoa.[1][2] Copro-Ag testing has been shown to achieve a sensitivity of 98% and specificity of 99% compared to the sensitivity of 38% with direct microscopy.[1][2] For species-specific diagnosis, PCR for gene sequences of T. solium achieves 100% specificity and 97% to 100% sensitivity. Serum testing includes an immunoblot assay for antibody detection for T. solium excretory-secretory (TSES) antigen has 95% sensitivity and 100% specificity.[1]
Diphyllobothriasis also relies on stool examination for distinct eggs, which can classify the genus but not the species.[7] Molecular methods, such as PCR of stool samples is the most reliable method to identify at the species level.[5] There is no reliable serologic testing to aid in the diagnosis. For both Diphyllobothrium and Taenia, there is no clinical reason to distinguish between species since treatment is the same; however, identifying the distinct species will aid in the epidemiologic picture of transmission and imported cases.
Since cysts in most tissues are asymptomatic or destroyed, they are rarely noticed or diagnosed. However, NCC leads to visible cystic lesions in the CNS and significant and variable symptomatology. A large part of NCC pathology is due to the host immune response to the T. solium larval cyst.[9] Once the cyst is in the tissue, the host immune system induces granuloma formation and perilesional edema. In the initial stage of NCC, there is a viable cyst with minimal pericystic inflammation. As the host immune response increases, the perilesional inflammation will kill the cyst, the fluid inside the cyst becomes dense, and the cyst shrinks until it is eventually cleared or replaced with residual calcification.[4] MRI and CT can identify calcific granulomatous "ring-enhancing" lesions found in the brain.[9] The diagnosis of NCC depends on radiologic suspicion and congruent interpretation of serology. Radiology can stage degeneration and the degree of inflammation but is also used to monitor the effectiveness of treatment.[3] Serology aids in the confirmation of the diagnosis.
Serology for NCC includes antibody and antigen detection through serum and CSF samples, respectively. Although antigen detection is possible with serum and urine, it was found to be higher in CSF.[4] Enzyme-linked immunoelectrotransfer blot assay (EITB) of serum for antibody detection against T. solium cysts has a 98% to 100% specificity in patients with more than one viable brain cyst; however, EITB has poor sensitivity for only one brain lesion.[4][9] Antibody detection does not indicate active cysticercosis since this can result from exposure alone and infections that did not establish or have resolved. The lifespan of the antibodies is variable and depends on the immune history of the host and the burden of infection.[4] Some patients with a history of multiple cysts can have positive antibodies for years after successful treatment. Monoclonal-based ELISA testing for antigen detection can demonstrate the presence of live parasites. Antigen levels drop quickly after treatment while antibodies may remain detectable.[4] Converting to negative antibodies is rare to occur in less than 1 year, but it is a marker of cure.
In general, positive antibody and antigen testing for NCC suggests a viable infection. A single lesion on imaging (or low infection burden) may test negative for both. Patients with only calcified lesions will typically be negative for antigens but may be negative or positive for antibodies. If imaging shows only calcified lesions and the patient has a positive antigen test, one should suspect a viable lesion that has been missed. Subarachnoid NCC is associated with high levels of antibody and antigen. Therefore, positive imaging with negative or weakly positive results should raise questions about the diagnosis.[4]
Treatment / Management
Treatment of adult Taenia tapeworm is responsive to common antihelmintic drugs; niclosamide, praziquantel, tribendimidine, and albendazole.[1] Praziquantel and niclosamide have been the treatment of choice[9]; however, praziquantel is the most cost-effective. Both praziquantel and albendazole cross the blood-brain-barrier while niclosamide does not. Crossing the BBB may cause neurologic consequences due to activation of undiagnosed latent NCC.[1] Treatment by endoscopic removal of the tapeworm is neither typical nor required for T. solium; however, has been performed in conjunction with praziquantel with good results.[10] Side effects for praziquantel are usually mild and do not require treatment but may be more frequent depending on the worm burden. Side effects include malaise, headache, dizziness, abdominal discomfort, nausea, temperature elevation, and rarely urticaria.[5] Treatment for both Taenia and Diphyllobothrium in adults or children is with a single 5- to 10-mg/kg dose of praziquantel.[6]
NCC treatment is based on the location of brain involvement and the number of cysts and may include symptomatic therapy (antiepileptics), antiparasitic therapy, or surgery. Often more than one is needed. Of note, antiparasitic drugs can also lead to temporary inflammation and increased symptoms; therefore, caution must be exercised.[3] One or two parenchymal lesions may be treated with short courses of albendazole and corticosteroids, while multiple lesions require combination albendazole and praziquantel with steroids.[11] Ventricular cysts are often surgically removed, and subarachnoid cysts may require longer courses of antiparasitic and anti-inflammatories.[11]
Differential Diagnosis
Given the asymptomatic or mild presentation and a wide range of symptoms for adult tapeworm infections, the diagnosis requires high clinical suspicion based on the patient's travel or exposure history of endemic regions. Patients in endemic regions who present with abdominal pain, diarrhea, or constipation merit stool microscopy. Given the low sensitivity of stool microscopy, negative results should not eliminate tapeworm infection from the differential; however, a broad differential for abdominal pain should be maintained to include other infectious etiologies (bacterial diarrhea, viral syndromes) or chronic inflammatory disorders. Repeat testing or empiric treatment may be necessary.
In most cases, stool microscopy can correctly diagnose the genus of tapeworm; however, there has been misidentifications given the similar shape, size or appearance of various parasitic eggs.[5] Newer stool molecular testing also may have cross-reactivity with other parasites or protozoa.[1][2] It is important to distinguish between the Taenia species as T. solium is at risk for causing neurocysticercosis while T. saginata is not.[8]
As stated above for NCC, this diagnosis requires imaging and serology interpretation. The differential for multiple ring-enhancing lesions of the brain is broad, to include bacterial (abscess, tuberculosis, syphilis), fungal (coccidioidomycosis, cryptococcosis, aspergillosis), parasitic (toxoplasmosis, echinococcosis), neoplastic (metastasis, CNS lymphoma), and inflammatory (multiple sclerosis, sarcoidosis).[12]
Prognosis
Adult tapeworms are responsive to anti-helminthic treatment, and patients have a good prognosis with complete resolution of symptoms. The largest concern is reinfection or undiagnosed parasites leading to complications or malnutrition.
NCC has a good prognosis with symptoms improving after treatment, but prognosis varies depending on the location and burden of disease. Subarachnoid and intraventricular cysts have significant morbidity and mortality while single parenchymal brain lesions have a high chance of survival with no seizure relapses.[3] Multiple cysts can be lethal or cause recurrent seizures.
Complications
Although rare, there are potential complications from the adult tapeworm infection mostly due to the worm migrating within the gastrointestinal tract. Complications include pancreatitis, cholecystitis (impacted worm in gallbladder/common bile duct), Meckel's diverticulitis, and bowel obstruction which may require surgical intervention.[2] As stated above, Diphyllobrothrium will absorb B12 from the gut lumen, and patients are at risk of developing pernicious anemia.[6] NCC complications include epilepsy, headache, neurologic deficits, strokes, and hydrocephalus.
Deterrence and Patient Education
Sanitation, meat inspection, and preparation are the cornerstone to preventing human infection of all three tapeworms. Since a single tapeworm can produce thousands of eggs per day, the environment can be easily contaminated if lacking basic hygiene.[5] Also, most species can mature in non-human hosts, maintaining their life cycle. Therefore, the treatment of human disease does not eliminate the parasite from endemic areas.
The best prophylaxis for Diphyllobothrium is to avoid consumption of raw, smoked, or pickled fish. Fish should be well cooked or alternatively frozen for 24 to 48 hours at -18 C. The FDA recommends that fish intended to be consumed raw should be blast frozen to -35 C or below for 15 hours or frozen to -20 C or below for 7 days.[5] Smoked salmon is brined before smoking in the United States, therefore, is not considered a source of infection.[6]
The current practice to prevent T. solium in pigs includes visual inspection of the meat for cysticerci. However, some studies suggest that inspection alone is not sufficient for control of bovine cysticercosis.[2] Molecular methods are being used to screen herds, but some tests (such as antibody ELISA) only indicate prior rather than active infection. Adequately cooking or freezing beef or pork prevents infections by Taenia. T. solium is killed by cooking pork to an internal temp of 65C (150F) or freezing at -20 C (-38 F) for at least 12 hours. Neither pickling nor salt-curing pork ensures infection prevention. For T. saginata, cook beef to an internal temperature of 56C (131F) or freeze at -10 C for 5 days. Pickling beef in 25% brine for 5 to 6 days is believed to render beef safe.[6]
Enhancing Healthcare Team Outcomes
Treatment and management of neurocysticercosis require a team-based healthcare approach. NCC will often require neurology and infectious disease consultation but depending on the location of the lesions may also incorporate neurosurgery evaluation. NCC can present in any patient population and can sometimes mimic preeclampsia in pregnant women. There have been several case studies of pregnant women with NCC presenting with seizures or headaches.[13] [Level 5] The care for these patients involves multiple specialties to include perinatologists, neurosurgery, infectious disease specialists, and nursing. The transition to discharge must also be considered to involve case management for potential psychosocial concerns. Because treatment is often very costly, this can have a significant impact on the patient's financial wellbeing. Therefore, the management of NCC goes beyond an interprofessional approach between specialists, but also into the psychosocial care for patients and even into the public sector for disease prevention and patient education.
Review Questions

Figure
CT Head neurocysticercosis. Contributed by Scott Dulebohn, MD

Figure
Photomicrograph, Brain tissue specimen revealed the presence of cysticerci, Cysticercosis, Eggs of a pork tapeworm, Taenia solium, Infestation of Brain Tissue, Neurocysticercosis, Parasitism, Pathology. Contributed by Dr. George R. Healy, The Centers (more...)

Figure
cysticercosis CT scan brain. Image courtesy S Bhimji MD

Figure
Scolex of the Taenia Saginata and Taenia Solium, Pork Tapeworm (top) and Beef Tapeworm (bottom). Contributed by Dr. Mae Melvin; The Centers for Disease Control and Prevention (CDC)

Figure
Diphyllobothrium latum. Image courtesy: https://www.cdc.gov/parasites/diphyllobothrium/index.html
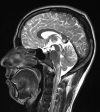
Figure
Neurocysticercosis in the CSF pathways. Contributed by Sunil Munakomi, MD
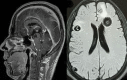
Figure
Disseminated neurocysticercosis. Contributed by Sunil Munakomi, MD
References
- 1.
-
Okello AL, Thomas LF. Human taeniasis: current insights into prevention and management strategies in endemic countries. Risk Manag Healthc Policy. 2017;10:107-116. [PMC free article: PMC5461055] [PubMed: 28615981]
- 2.
-
Silva CV, Costa-Cruz JM. A glance at Taenia saginata infection, diagnosis, vaccine, biological control and treatment. Infect Disord Drug Targets. 2010 Oct;10(5):313-21. [PubMed: 20701576]
- 3.
-
Garcia HH. Neurocysticercosis. Neurol Clin. 2018 Nov;36(4):851-864. [PubMed: 30366559]
- 4.
-
Garcia HH, O'Neal SE, Noh J, Handali S., Cysticercosis Working Group in Peru. Laboratory Diagnosis of Neurocysticercosis (Taenia solium). J Clin Microbiol. 2018 Sep;56(9) [PMC free article: PMC6113464] [PubMed: 29875195]
- 5.
-
Scholz T, Garcia HH, Kuchta R, Wicht B. Update on the human broad tapeworm (genus diphyllobothrium), including clinical relevance. Clin Microbiol Rev. 2009 Jan;22(1):146-60, Table of Contents. [PMC free article: PMC2620636] [PubMed: 19136438]
- 6.
-
Tanowitz HB, Weiss LM, Wittner M. Tapeworms. Curr Infect Dis Rep. 2001 Feb;3(1):77-84. [PubMed: 11177734]
- 7.
-
Kuchta R, Brabec J, Kubáčková P, Scholz T. Tapeworm Diphyllobothrium dendriticum (Cestoda)--neglected or emerging human parasite? PLoS Negl Trop Dis. 2013;7(12):e2535. [PMC free article: PMC3873255] [PubMed: 24386497]
- 8.
-
Dermauw V, Dorny P, Braae UC, Devleesschauwer B, Robertson LJ, Saratsis A, Thomas LF. Epidemiology of Taenia saginata taeniosis/cysticercosis: a systematic review of the distribution in southern and eastern Africa. Parasit Vectors. 2018 Nov 06;11(1):578. [PMC free article: PMC6219070] [PubMed: 30400948]
- 9.
-
Reddy DS, Volkmer R. Neurocysticercosis as an infectious acquired epilepsy worldwide. Seizure. 2017 Nov;52:176-181. [PubMed: 29055271]
- 10.
-
Philips CA, Sahney A. Taenia solium. N Engl J Med. 2017 Jan 26;376(4):e4. [PubMed: 28121503]
- 11.
-
Webb CM, White AC. Update on the Diagnosis and Management of Neurocysticercosis. Curr Infect Dis Rep. 2016 Dec;18(12):44. [PubMed: 27787774]
- 12.
-
Garg RK, Sinha MK. Multiple ring-enhancing lesions of the brain. J Postgrad Med. 2010 Oct-Dec;56(4):307-16. [PubMed: 20935408]
- 13.
-
Webb C, Rosa M, Olson G, Cabada M. Neurocysticercosis in Pregnancy. AJP Rep. 2018 Apr;8(2):e51-e56. [PMC free article: PMC5891319] [PubMed: 29637011]
Source: https://www.ncbi.nlm.nih.gov/books/NBK537154/